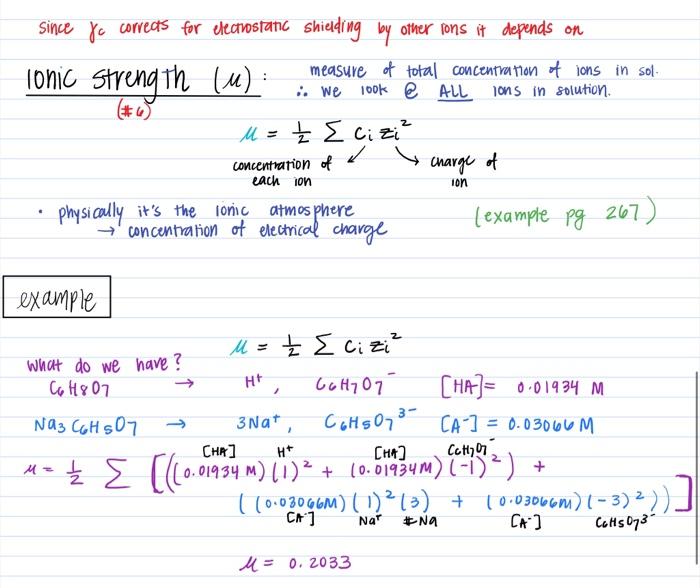
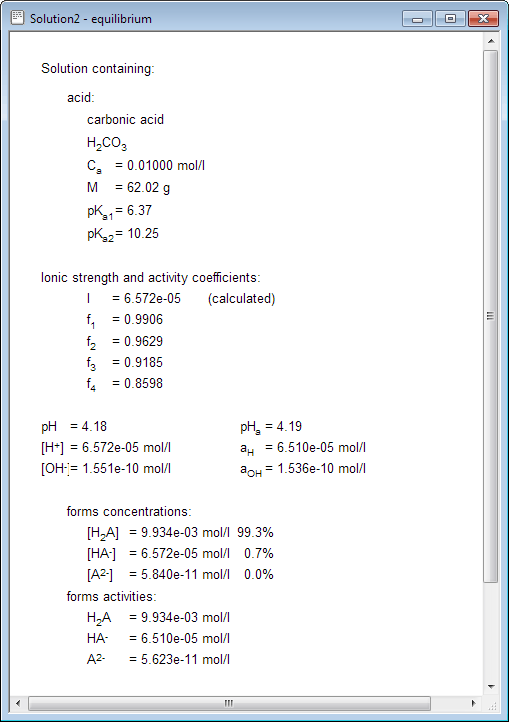
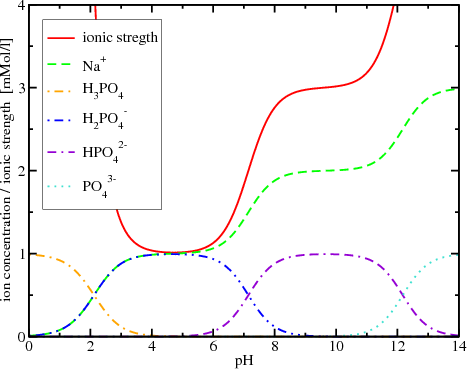
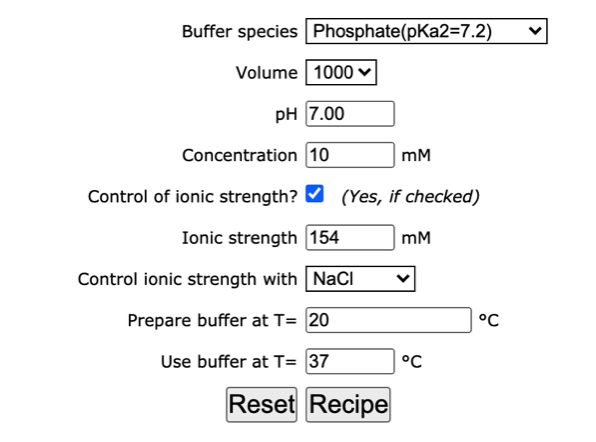
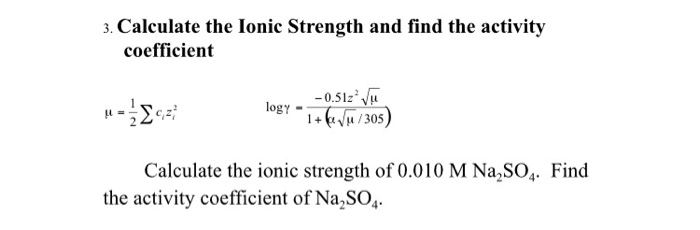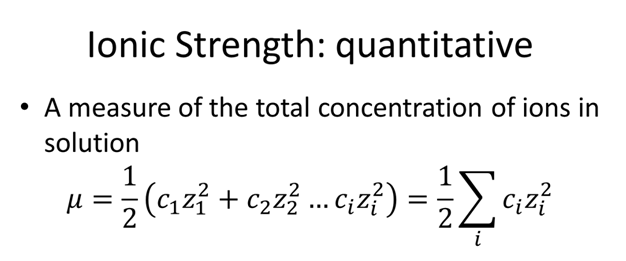physical chemistry - Calculating the ionic strength of a histidine solution - Chemistry Stack Exchange
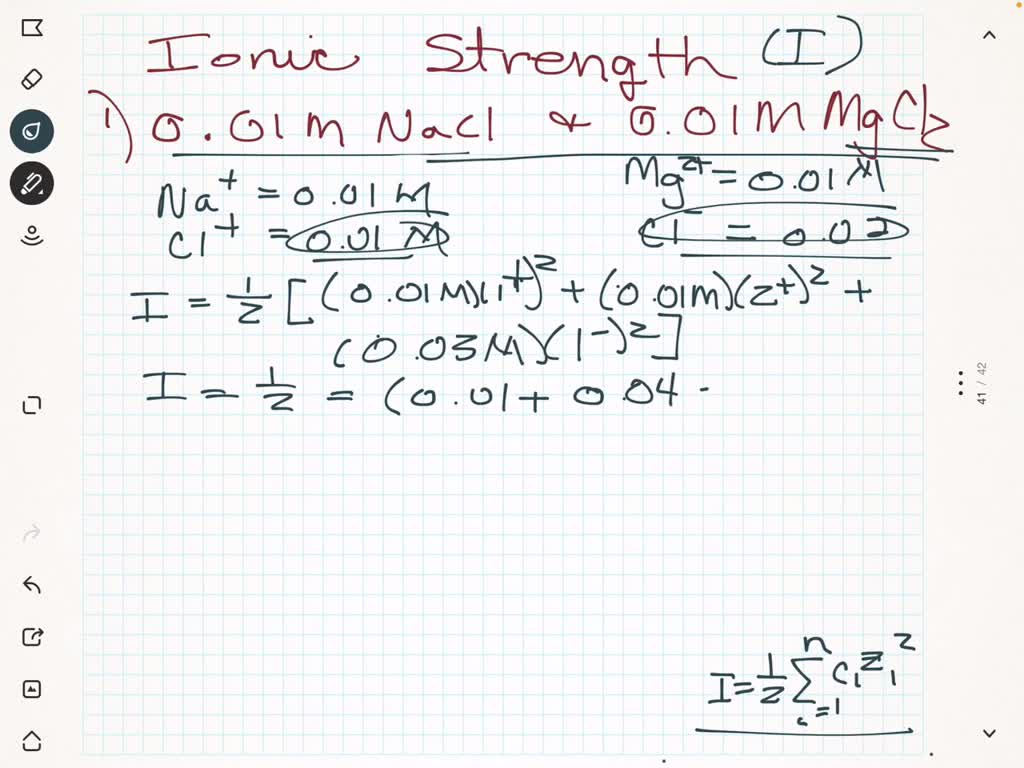
SOLVED: Which solution below has the highest ionic strength? 0.01 M NaCl and 0.01 M MgCl2 0.02 M NaCl 0.02 M MgCl2 They - will all have the same ionic strength:

Ionic strength - Solved problems-electrochemistry-calculation-example-IIT JEE NEET JAM CSIR NET GATE - YouTube

Find the ionic strength of (Electrochemistry): i. 0.05 \ mol \ dm^{-3} \ KCl(aq) ii. 0.05 \ mol \ kg^{-1} LaFe(CN)_6(ag) Give detail explanation. (eq. why does CN have a charge of

Find the ionic strength of (Electrochemistry): i. 0.05 \ mol \ dm^{-3} \ KCl(aq) ii. 0.05 \ mol \ kg^{-1} LaFe(CN)_6(ag) Give detail explanation. (eq. why does CN have a charge of

Find the ionic strength of (Electrochemistry): i. 0.05 \ mol \ dm^{-3} \ KCl(aq) ii. 0.05 \ mol \ kg^{-1} LaFe(CN)_6(ag) Give detail explanation. (eq. why does CN have a charge of
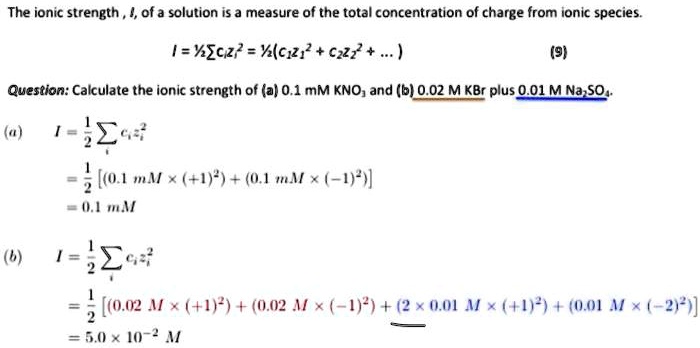
SOLVED: The ionic strength of a solution is a measure of the total concentration of charge from ionic species. (a) Calculate the ionic strength of 0.1 mM KNO3: I = (0.1 mM * (+